News
Intelligent Upgrade: The New Infrastructure for the Development of Evidence-Based Traditional Chinese Medicine
On March 6, the general AI agent Manus, developed by a Chinese team, sparked widespread discussion across the internet with its “human-level task execution capabilities”. As researchers in the field of evidence-based traditional Chinese medicine (TCM), we see more than just AI’s ability to generate PPTs or write code—we recognize it as an accelerating engine for the intelligent transformation of the standardized evidence-based TCM ecosystem. The true value of Manus lies not in replacing human intelligence but in leveraging massive data processing and logical inference to inject new momentum into the generation, integration, and decision-making of TCM evidence. Currently, AI-assisted clinical trial design and automatic systematic review generation have already emerged in the global medical community. To tackle the dual challenges of internationalization and scientific advancement, the field of evidence-based TCM urgently needs to establish an independent technology-driven system.
1.Evidence Generation: The Precision Revolution in TCM Clinical Trials
As a WHO-recognized primary registry, ITMCTR serves as a global hub for traditional medicine research, currently housing 560,000 clinical trial records. The integration of large language models is set to transform the implementation paradigm of TCM clinical research, driving the generation of high-quality evidence in evidence-based TCM.
As a WHO-recognized primary registry, ITMCTR serves as a global hub for traditional medicine research, currently housing 560,000 clinical trial records. The integration of large language models is set to transform the implementation paradigm of TCM clinical research, driving the generation of high-quality evidence in evidence-based TCM.

Intelligent Design Optimization: By deeply analyzing the extensive global traditional medicine trial data accumulated on the platform, AI can build a cross-language, cross-regional trial design knowledge base to provide intelligent assistance for researchers. For example, when a user submits a draft protocol for an acupuncture trial on chronic low back pain, the system can automatically compare it with globally registered or published studies, identify common issues such as insufficient sample size or lack of blinding, and generate optimization suggestions for composite endpoints by integrating recommendations from both Western and TCM clinical guidelines.
Instant Compliance Review: For platform administrators, manually reviewing 30,000 trial-related records per month is highly time-consuming. With the batch auditing capabilities of large language models, compliance review efficiency can be increased by 5–8 times. For instance, the system can automatically check whether the intervention descriptions in acupuncture clinical trials align with STRICTA standards or detect logical inconsistencies between “randomized grouping” statements and actual blinding implementation. This significantly reduces the time required for researchers to obtain clinical trial registration numbers.
Dynamic Quality Control: During the implementation phase after trial registration, the system can track patient enrollment progress across study sites, monitor data submission quality, and assess research outcomes in real-time. Through natural language processing, it can automatically extract efficacy data from published papers and compare them with the original trial objectives, providing precise targets for subsequent quality improvements.
2. Evidence Integration: A Comprehensive Overview of the Clinical Value of TCM
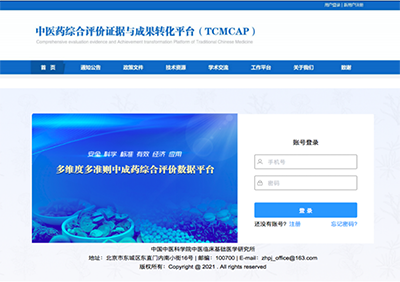
The Comprehensive Evaluation Evidence and Achievement Transformation Platform of TCM (TCMCAP) is designed to support evidence-based clinical comprehensive evaluation research of traditional Chinese patent medicines. The platform integrates multiple functions, including registration for comprehensive evaluation of TCM, evidence collection related to TCM clinical assessments, data storage, professional review, data analysis and processing, model calculation, result visualization, and industry trend sharing. Since the comprehensive evaluation of each traditional Chinese patent medicine requires processing an average of over 300 texts, there is an urgent need to overcome the bottleneck of data integration and deep analysis.
The Comprehensive Evaluation Evidence and Achievement Transformation Platform of TCM (TCMCAP) is designed to support evidence-based clinical comprehensive evaluation research of traditional Chinese patent medicines. The platform integrates multiple functions, including registration for comprehensive evaluation of TCM, evidence collection related to TCM clinical assessments, data storage, professional review, data analysis and processing, model calculation, result visualization, and industry trend sharing. Since the comprehensive evaluation of each traditional Chinese patent medicine requires processing an average of over 300 texts, there is an urgent need to overcome the bottleneck of data integration and deep analysis.
Multi-source Evidence Structuring: Large language models significantly improve data efficiency by automating the extraction and structuring of multi-source evidence (such as clinical guidelines and case reports). They can accurately extract key information such as indications and efficacy indicators from unstructured text, assisting the double-blind data extraction process and reducing human error.
Cross-modal Knowledge Network: Leveraging cross-modal association capabilities, large language models can construct a “disease-drug-evidence” network, integrating evidence from ancient texts, clinical trial results, and economic evaluations. This enhances the interpretability of the ED-CEM-CPM evaluation model.
Intelligent Report Generation: Based on the PRICE-CPM template for TCM comprehensive evaluation reports, the system automatically generates customized evaluation reports. These reports precisely address the differentiated needs of medical institutions, pharmaceutical companies, and regulatory bodies (such as emphasizing quality standards important to enterprises), achieving full-chain effectiveness from evidence analysis to policy implementation.
3.Evidence-based Decision Making: The Accelerator for the Development and Translation of TCM Standards
The Standards and Guidelines Information Service Platform of TCM has comprehensively archived nearly 3,000 national standards, group standards, guidelines, and international standards for TCM, creating an authoritative TCM standards database. It also enables the supervision and management of TCM group standards. With the integration of large language models, the platform will achieve a three-fold leap:
The Standards and Guidelines Information Service Platform of TCM has comprehensively archived nearly 3,000 national standards, group standards, guidelines, and international standards for TCM, creating an authoritative TCM standards database. It also enables the supervision and management of TCM group standards. With the integration of large language models, the platform will achieve a three-fold leap:
Dynamic Standard Updates:By integrating large language models, the platform can continuously monitor domestic and international TCM standard information in real-time, accurately identify relevant standard entries, and automatically update them in the knowledge base. Additionally, based on the structural characteristics of historical standard documents, the system can generate draft frameworks to assist users in drafting initial versions of standards.
Intelligent Text Review:In the standard intelligent evaluation process, large language models deeply analyze specifications such as GB/T 1.1. The system can not only detect issues in wording (such as logical ambiguities or vague expressions) but also construct multi-level format compliance detection models (such as clause numbering systems and appendix citation norms). By calculating semantic similarity, the system provides dynamic scoring and iterative optimization suggestions.
Decision Support Empowerment: For standard decision support, large language models can create a “standard-treatment pathway-efficacy evidence” knowledge graph, enabling intelligent mapping of standard texts to application scenarios (e.g., automatically matching standards for Chinese medicine preparation processes, drug quality control indicators, and compatibility warnings). In clinical applications, the system can generate personalized treatment recommendations through natural language interactions, simultaneously linking relevant training resources and methodological guidelines from the platform. This promotes the transformation of TCM standards from static texts to dynamic clinical decision-making tools.
Conclusion
The modernization of TCM is currently facing three major challenges: fragmented multi-source evidence, lagging standard iterations, and inefficient clinical translation. The intelligent evidence ecosystem, built by large language models, empowers the entire chain of evidence generation, integration, and decision-making. This not only breaks down data silos and improves research efficiency but also transforms standardized outcomes into dynamic clinical guidelines. When the AI potential showcased by Manus deeply aligns with the evidence-based needs of TCM, a transformation driven by technology to upgrade traditional medicine has quietly begun.
The modernization of TCM is currently facing three major challenges: fragmented multi-source evidence, lagging standard iterations, and inefficient clinical translation. The intelligent evidence ecosystem, built by large language models, empowers the entire chain of evidence generation, integration, and decision-making. This not only breaks down data silos and improves research efficiency but also transforms standardized outcomes into dynamic clinical guidelines. When the AI potential showcased by Manus deeply aligns with the evidence-based needs of TCM, a transformation driven by technology to upgrade traditional medicine has quietly begun.
Team Introduction
The TCM Standards and Evidence-Based Team is led by Prof Huang Luqi and is dedicated to key common technologies and empirical research within the full evidence chain of TCM. The team is responsible for the operation of ITMCTR, the Comprehensive Evaluation Evidence and Achievement Transformation Platform of TCM, and the Standards and Guidelines Information Service Platform of TCM. The team is based at China Center for Evidence Based Traditional Chinese Medicine, the world’s first evidence-based medicine center in the field of TCM. In collaboration with leading institutions in the field, the team conducts research on TCM evidence-based medicine, consensus on methodologies, standard development, clinical evaluation, evidence sample management, and evidence information services.
The TCM Standards and Evidence-Based Team is led by Prof Huang Luqi and is dedicated to key common technologies and empirical research within the full evidence chain of TCM. The team is responsible for the operation of ITMCTR, the Comprehensive Evaluation Evidence and Achievement Transformation Platform of TCM, and the Standards and Guidelines Information Service Platform of TCM. The team is based at China Center for Evidence Based Traditional Chinese Medicine, the world’s first evidence-based medicine center in the field of TCM. In collaboration with leading institutions in the field, the team conducts research on TCM evidence-based medicine, consensus on methodologies, standard development, clinical evaluation, evidence sample management, and evidence information services.